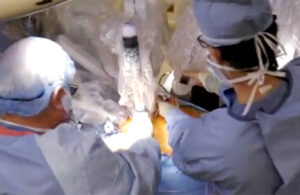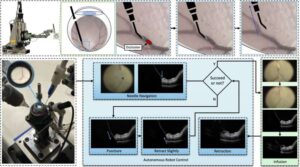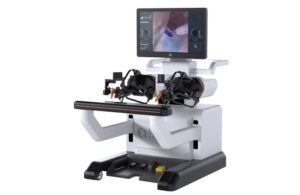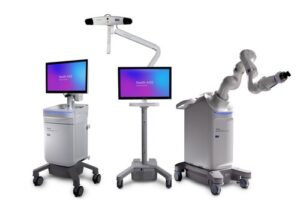
Medtronic receives FDA clearance for Stealth AXiS™ surgical system, first integrated planning, navigation and robotics platform for spine surgery
GALWAY, Ireland, Feb. 13, 2026 /PRNewswire/ — Medtronic (NYSE: MDT), a global leader in healthcare technology, today announced U.S. Food and Drug Administration (FDA) clearance of the Stealth AXiS™ surgical system, a next-generation platform that brings planning, navigation, and robotics together into a single, intelligent system for spine surgery.