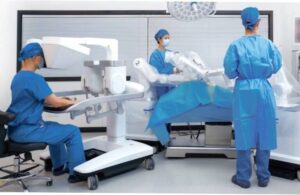
Ronovo Surgical wins regulatory nod for laparoscopic surgical robot in China
Ronovo Surgical has received regulatory approval for its Carina surgical robotic platform in China.

Ronovo Surgical has received regulatory approval for its Carina surgical robotic platform in China.

Researchers at UC San Francisco have enabled a man who is paralyzed to control a robotic arm through a device that relays signals from his brain to a computer.

Capstan Medical today announced the first successful transcatheter mitral valve replacements using its novel implant and surgical robotics system.

Phantom Neuro today announced it received FDA breakthrough device designation and Targeted Acceleration Pathway (TAP) designation for its minimally invasive neural interface Phantom X.

Vitestro today launched its Aletta autonomous robotic phlebotomy device (ARPD) that performs automated blood draws.
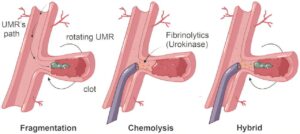
Researchers at the TechMed Center of the University of Twente and Radboud University Medical Center have removed blood clots with wireless magnetic robots. This innovation promises to transform treatment for life-threatening vascular conditions like thrombosis.
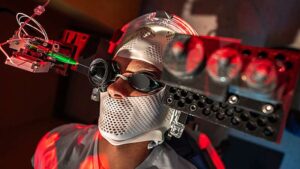
When even the most highly trained surgeons perform procedures on the retina—one of the smallest, most delicate parts of the human body—the stakes are high. Surgeons must account for patients’ breathing, snoring, and eye movements, along with their own involuntary hand tremors, while they work on a layer of cells less than a millimeter thick.

A team of UC Berkeley engineers from the Embodied Dexterity Group has developed a wearable device to enhance grasping functionality in this population. Dubbed the Dorsal Grasper, this assistive device leverages voluntary wrist extension and uses supernumerary robotic fingers on the back of the hand to facilitate human-robot collaborative grasping.

Stereotaxis (NYSE:STXS) said today that it has secured CE mark approval for its Magic robotically-navigated magnetic ablation catheter.

Biobot Surgical announced today that it received CE mark for its Mona Lisa 2.0 surgical robotic platform for urology applications.