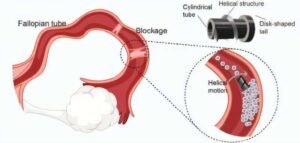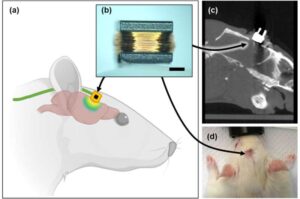
BAIBYS™ Announces a Breakthrough in Fertility Treatment with a Newly CE Marked IVF System
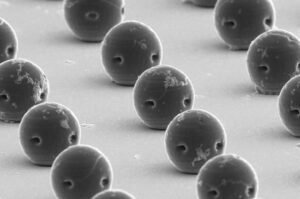
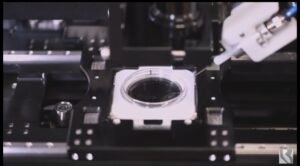
TEL AVIV, Israel, Jan. 14, 2025 /PRNewswire/ — BAIBYS™, a pioneering Israeli startup in AI-powered micro-robotics for Artificial Reproductive Technology (ART), today announced it has received the CE mark for its innovative BAIBYS™ system.