
Intuitive wins expanded FDA indications for da Vinci SP for trio of new procedures
Intuitive (NASDAQ: ISRG)+
announced today that the FDA cleared its da Vinci single port (SP) surgical robot for use in new types of procedures.

Intuitive (NASDAQ: ISRG)+
announced today that the FDA cleared its da Vinci single port (SP) surgical robot for use in new types of procedures.
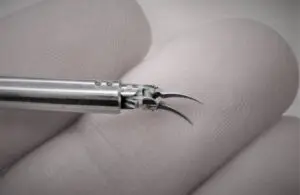
Medical Microinstruments (MMI) announced today that the FDA granted 510(k) clearance for its NanoWrist surgical robot instruments.
SS Innovations (Nasdaq:SSII) announced today that it submitted a 510(k) premarket notification to the FDA for its Mantra surgical robot.

Partnership will expand Shoulder Innovations’ Disruptive Ecosystem with Advanced Enabling Technology, Complementing Surgeon and Patient Needs in the ASC
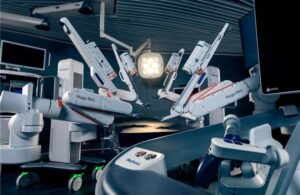
Medtronic (NYSE: MDT)+
announced today that the FDA cleared its Hugo robotic-assisted surgery system for use in urologic surgical procedures.

Medical Microinstruments (MMI) announced today that it won reimbursement for its surgical lymphovenous bypass (LVB) surgery system.
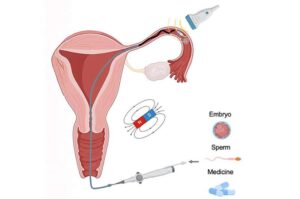
A new international study led by the Nanobiosystems group at CIC nanoGUNE, is developing miniature, non-invasive, precise robotic catheters for use in reproductive medicine and gynecological health.

Microbot is collaborating with Emory to establish an Endovascular Robotics Program in interventional radiology to enhance the growing and evolving specialty field.

What if a robot could show us how the brain keeps us balanced? UBC scientists built one—and their discovery could help shape new ways to reduce fall risk for millions of people.
RESTON, Va., Nov. 25, 2025 /PRNewswire/ — VSI® Spine Surgeon Dr. Christopher Good has achieved a historic milestone in robotic spine surgery, performing the world’s first minimally invasive robotic Bertolotti’s resection surgery at Reston Hospital Center (HCA Virginia Health System). This first-ever procedure introduces a transformative surgical option for patients suffering from chronic low back pain caused by Bertolotti syndrome, an underdiagnosed spinal condition affecting an estimated 4-8% of the population.