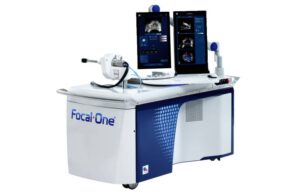
EDAP wins FDA nod for robotic high-intensity focused ultrasound system workflows
EDAP TMS SA (Nasdaq:EDAP) announced today that the FDA granted 510(k) clearance for new workflows for its ultrasound technology.

EDAP TMS SA (Nasdaq:EDAP) announced today that the FDA granted 510(k) clearance for new workflows for its ultrasound technology.
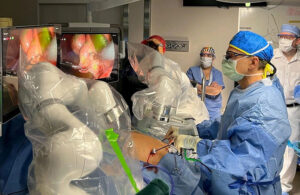
Levita Magnetics has received FDA 510(k) clearance for its Magnetic Surgical System (MSS) to be used in certain pediatric surgeries, with the first U.S. surgery performed at Cleveland Clinic Children’s earlier this month.

A decade ago, at age 55, Don Lewis suffered a stroke in his sleep. When he woke up, he couldn’t move his left arm or leg. Lewis’s neighbor realized his truck hadn’t moved in two days and called 911 for a welfare check. When paramedics found him, he was paralyzed on one side.
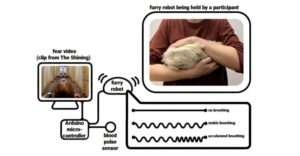
Humans can “catch” fear from robots, new research has shown. The findings—by a team of psychologists from the University of Amsterdam and the University of British Columbia—shed new light on how emotions can spread through touch, with implications for human relationships, mental health, and future technologies such as virtual reality and wearable devices.
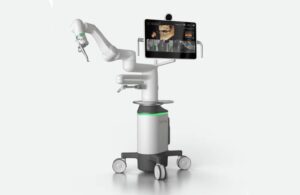
Neocis announced today that it launched Yomi S, its next-generation robotic platform for dental implant surgery.

Competing with companies including Stryker and J&J, Zimmer has moved to strengthen its position by developing an updated robot.
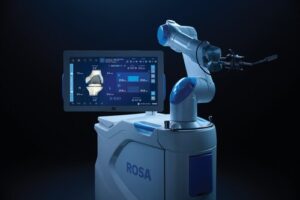
WARSAW, Ind., Nov. 14, 2025 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global medical technology leader, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of ROSA® Knee with OptimiZe™, an enhanced version of its ROSA® Knee System that offers a more customized experience for surgeons to help deliver accurate and reproducible outcomes1 in robotic-assisted total knee replacement surgery.
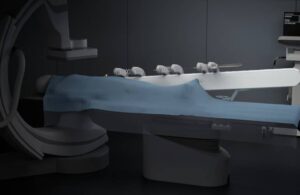
Sentante today announced the completion of a first-of-its-kind remote stroke procedure in Scotland using its robotic platform.
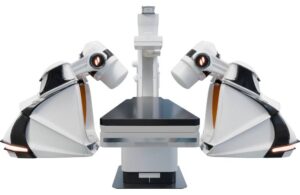
Stereotaxis (NYSE:STXS) announced today that it received FDA 510(k) clearance for its next-generation GenesisX surgical robot.
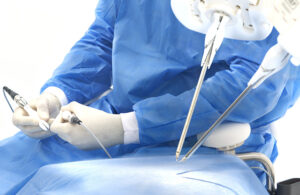
Medical Microinstruments (MMI) announced today that it received FDA investigational device exemption (IDE) to study its microsurgery platform