
New Cannibalistic Robots Consume Other Machines to Grow and Heal on Their Own
Learn how “robot metabolism” allows machines to take material from their surroundings to “grow” and to “heal.”

Learn how “robot metabolism” allows machines to take material from their surroundings to “grow” and to “heal.”

Sculpteo has announced a collaboration with My Human Kit, a French association specialising in inclusive innovation in the design and manufacture of personalised, accessible, and sustainable prosthetics.
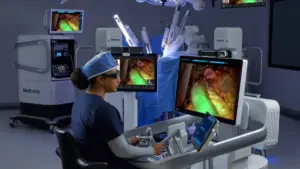
Medtronic said its market-leading LigaSure technology can now be used with its Hugo soft tissue robotic surgery system in Europe.

LivsMed today introduced Stark, its new telesurgery-native robotic system for remote surgery in partnership with Sovato.

Intuitive Surgical (Nasdaq: ISRG)+
announced today that the FDA cleared the latest advanced energy instrumentation for its da Vinci systems.
The FDA has granted 510(k) clearance to the TaviPilot AI software developed by Caranx Medical, according to company officials.

Mendaera has received FDA 510(k) clearance for its Focalist handheld robotic system, which enhances the precision of ultrasound-guided needle placement across multiple specialties.
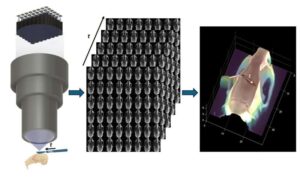
For over a century, surgeons performing delicate procedures have relied on stereoscopic microscopes to gain a sense of depth. These tools mimic human vision by presenting slightly different images to each eye, allowing the brain to perceive three-dimensional structures—a crucial aid when working with fragile blood vessels or intricate brain tissue. Despite modern upgrades like digital displays and video capture, today’s operating microscopes still depend on the same core principle: two views, interpreted by the human brain.
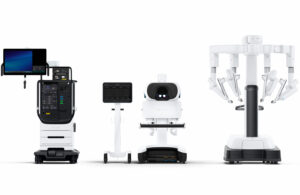
Intuitive Surgical (Nasdaq: ISRG)+
announced today that it received CE mark for its da Vinci 5 surgical robotic system.
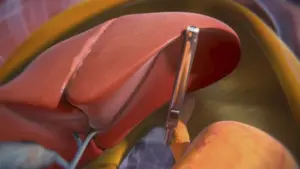
Magnetic-Assisted Robotic Platform Now Approved for Bariatric and Hiatal Hernia Procedures, Broadening Access to Scar-Reducing Surgical Innovation