
ChoiceSpine™ Launches Surgical App Integration with eCential Robotics Platform
New ChoiceSpine™ App Enhances Precision and Efficiency in Spine Surgery with Op.n™ Robotic and Navigation Technology

New ChoiceSpine™ App Enhances Precision and Efficiency in Spine Surgery with Op.n™ Robotic and Navigation Technology
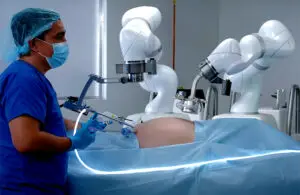
Levita Magnetics announced today that it received expanded FDA 510(k) clearance for its MARS (magnetic-assisted robotic surgery) system.

Brain Navi Biotechnology announced today that it received FDA 510(k) clearance for its NaoTrac stereotaxic guiding surgical robotic device.
Johnson & Johnson MedTech (NYSE: JNJ)+
today announced the U.S. launch of its Ethicon 4000 surgical stapler.

Distalmotion announced today that it received FDA 510(k) clearance for the use of its Dexter surgical robot in adult cholecystectomy (gallbladder removal).

The company said in a news release that the latest clearance marks a significant step forward in gastrointestinal (GI) motility monitoring. It offers clinicians an advanced, non-invasive tool for assessing whole-gut transit times with unmatched accuracy and patient comfort.

Virtuoso Surgical CEO Duke Herrell took to LinkedIn to announce successful first-in-human clinical cases with the company’s robotic endoscopy system.
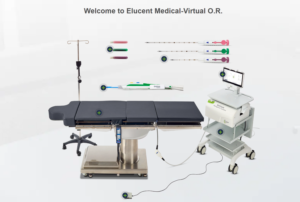
Elucent Medical announced today that it received FDA breakthrough device designation for its EnVisio X1 In-Body Spatial Intelligence system.
EndoQuest Robotics announced today that investigators completed the first procedures of the PARADIGM pivotal clinical trial of its surgical robot
Swiss Clinical Trial Marks Key Step in Global Expansion of Precision Hearing Implant Technology