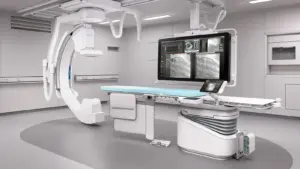
United Imaging Expands its U.S. Portfolio with the FDA Clearance of the uAngio® AVIVA for Interventional X-Ray
HOUSTON, May 12, 2025 /PRNewswire/ — United Imaging, a global manufacturer of modern medical imaging technology, announces that its first Interventional X-ray system is now FDA cleared. uAngio AVIVA is an intuitively bionic ceiling-mounted system that uses intelligent robotics, voice control, vision and imaging to serve as a critically important assistant to clinical staff in the interventional suite.