
J&J launches IVL device in Europe; Medtronic looks at pacing for more patients
J&J’s Shockwave Javelin intravascular lithotripsy catheter treats people with peripheral artery disease.

J&J’s Shockwave Javelin intravascular lithotripsy catheter treats people with peripheral artery disease.
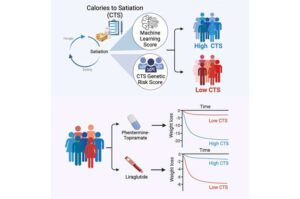
Mayo Clinic researchers have developed a genetic test that can help predict how people will respond to weight loss medications such as GLP-1s.
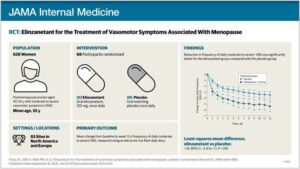
The investigational drug elinzanetant significantly reduces hot flashes and night sweats for postmenopausal women, a large, international clinical trial has found.
A team led by Christian Baumgartner of the Institute of Health Care Engineering at Graz University of Technology (TU Graz) has developed a highly detailed digital twin of the A549 lung cancer cell line. The twin builds on bioelectric processes and calcium dynamics in the cell interior in innovative new ways.
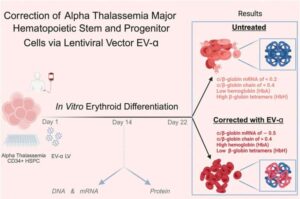
Thanks to in-utero blood transfusion technology, what was once a fatal diagnosis in the womb can now result in live births. However, this medical advancement created a new challenge: a growing population of children born with that diagnosis—the severe, inherited blood disorder alpha thalassemia—which requires lifelong specialized care.

Scientists have developed and tested a deep-learning model that could support clinicians by providing accurate results and clear, explainable insights—including a model-estimated probability score for autism.

Researchers led by Rice University’s Yong Lin Kong have developed a soft but strong metamaterial that can be controlled remotely to rapidly transform its size and shape.
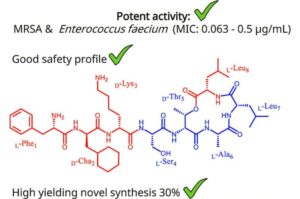
Scientists at the University of Liverpool, working with international collaborators, have discovered Novltex, a groundbreaking new class of antibiotics with potent activity against some of the world’s most dangerous multidrug-resistant (MDR) bacteria.
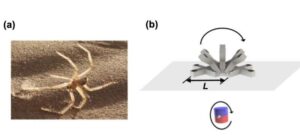
A team of researchers at the University of Macau in China recently developed new soft magnetic robots that can climb inverted surfaces and move in complex environments, which could allow them to deliver drugs to specific locations in the GI tract.
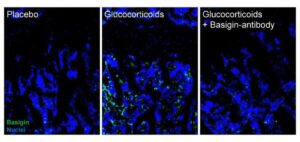
A new study from UC Davis Health researchers reveals a promising new way to treat various bone loss conditions, including age-related osteoporosis.