Dornier MedTech America launches Hoover™ and AXIS® II SLIM, raising the bar in kidney stone treatment
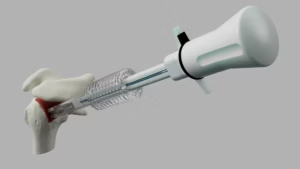
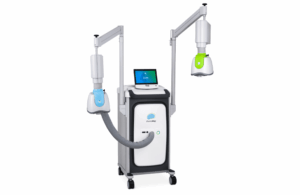
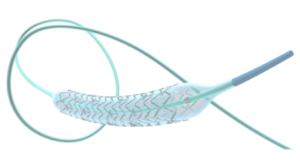
KENNESAW, Ga., Sept. 17, 2025 /PRNewswire/ — Dornier MedTech America (DMTA) has announced the full U.S. commercial launch of its Dornier Hoover Flexible and Navigable Suction Ureteral Access Sheath (FANS) and Dornier Axis II Slim single-use ureteroscope with a 5.6Fr. distal tip.