A ‘universal’ therapy against the seasonal flu? Antibody cocktail targets virus weak spot
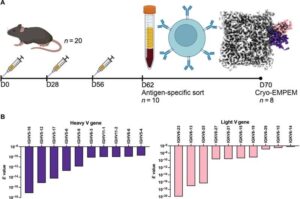
An unusual therapy developed at The Jackson Laboratory (JAX) could change the way the world fights influenza, one of the deadliest infectious diseases. In a new study in Science Advances, researchers report that a cocktail of antibodies protected mice—including those with weakened immune systems—from nearly every strain of influenza tested, including avian and swine variants that pose pandemic threats.