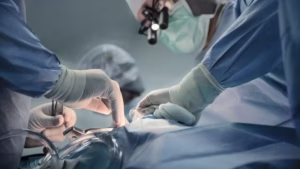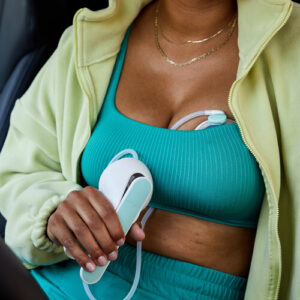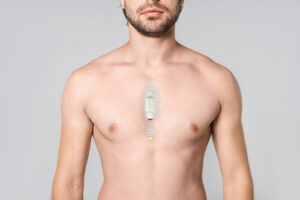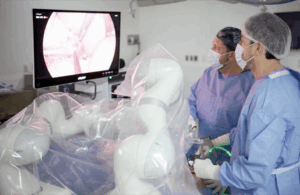
Levita Magnetics reports first AI-guided autonomous gall bladder surgery with MARS system
Levita Magnetics announced today that surgeons used its MARS platform to perform the world’s first gall bladder surgery featuring an autonomous surgical camera.