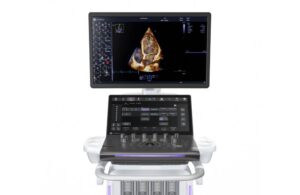
GE HealthCare launches new AI-powered cardiovascular ultrasound system
GE HealthCare (Nasdaq: GEHC)+
today announced the launch of Vivid Pioneer, its newest, most advanced cardiovascular ultrasound system.

GE HealthCare (Nasdaq: GEHC)+
today announced the launch of Vivid Pioneer, its newest, most advanced cardiovascular ultrasound system.
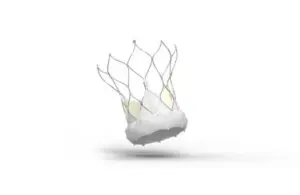
Abbott (NYSE: ABT)+
announced today that it received CE mark for an expanded indication for its Navitor TAVI system.
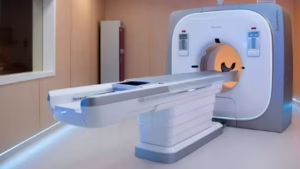
The NeuViz P10 uses a cadmium zinc telluride detector to directly convert X-ray photons, thereby eliminating light conversion.

A multi-institutional clinical trial conducted at the UNC Lineberger Comprehensive Cancer Center and 21 other U.S. sites found that a single administration of autologous tumor-infiltrating lymphocyte (TIL) cell therapy helped stabilize metastatic head and neck squamous cell carcinoma (HNSCC) in some patients. This finding is significant, as many of these patients had previously undergone multiple treatments without success.
Biomedical engineers at Duke University have successfully conducted experiments to treat damage caused by heart attacks in nonhuman primates using gene therapy for the first time.
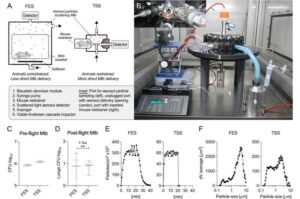
Tuberculosis has been a scourge upon humanity throughout history. In killing more than a million each year worldwide, it remains the leading cause of death from a single infectious pathogen.
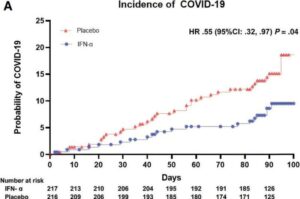
A world-first clinical trial has found that a simple daily nasal spray can significantly reduce the risk of COVID-19 in cancer patients, offering a potential new tool to protect vulnerable people from the virus.

A new diagnostic method would confirm sepsis infections earlier, cutting critical hours in the “race against time” to save patients’ lives.

Researchers have reported results from the first-ever clinical trial of a new class of targeted therapy in pet cats with head and neck squamous cell carcinoma (HNSCC)—a cancer which is notoriously deadly and difficult to treat.
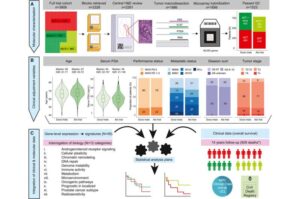
Testing the molecular profile of tumors identifies which patients with advanced prostate cancer are more likely to benefit from chemotherapy and live longer, sparing patients less likely to benefit from unpleasant side effects, according to a new study led by UCL researchers.