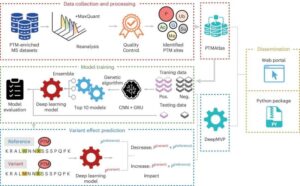
AI reveals how protein modifications link mutations to disease
Researchers at Baylor College of Medicine have developed an artificial intelligence (AI) model that reveals how protein modifications link genetic mutations to disease. The method, called DeepMVP and published in Nature Methods, significantly outperforms previously published models and has implications for the development of novel therapeutics.