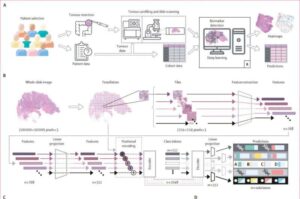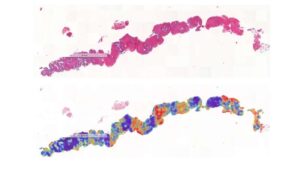
AI detects early prostate cancer in more than 80% of samples missed by pathologists
Men assessed as healthy after a pathologist analyzes their tissue sample may still have an early form of prostate cancer. Using AI, researchers at Uppsala University have been able to find subtle tissue changes that allow the cancer to be detected long before it becomes visible to the human eye.