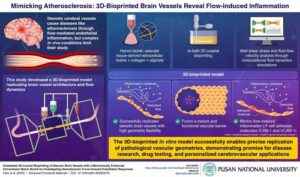
Pusan National University Unveils 3D-Printed Brain Vessels to Transform Atherosclerosis Research
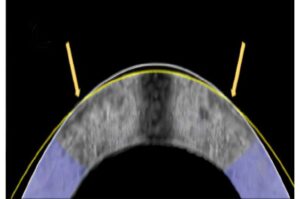
BUSAN, South Korea, Aug. 19, 2025 /PRNewswire/ — Cerebrovascular diseases, including atherosclerosis and stroke, remain a major global health concern. A common feature of these diseases is vascular stenosis—the narrowing of blood vessels—which disrupts normal blood flow and contributes to chronic inflammation in vessel walls