
Onward wins FDA IDE for spinal cord stimulation to address blood pressure instability
Onward Medical announced today that it received FDA investigational device exemption (IDE) for its ARC-IM system.

Onward Medical announced today that it received FDA investigational device exemption (IDE) for its ARC-IM system.
Would you swap your reading glasses for a single drop if it worked this fast?
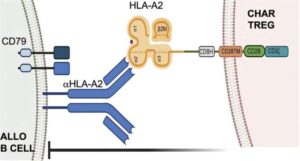
A Medical University of South Carolina team reports in Frontiers in Immunology that it has engineered a new type of genetically modified immune cell that can precisely target and neutralize antibody-producing cells complicit in organ rejection.
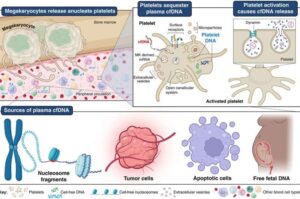
Swansea University has helped uncover a surprising new role for platelets—one that could significantly advance early cancer detection.
Researchers at Columbia Engineering have built a cancer therapy that makes bacteria and viruses work as a team. In a study published in Nature Biomedical Engineering, the Synthetic Biological Systems Lab shows how their system hides a virus inside a tumor-seeking bacterium, smuggles it past the immune system, and unleashes it inside cancerous tumors.
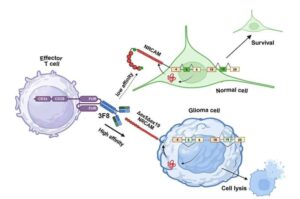
A new study, led by researchers at Children’s Hospital of Philadelphia (CHOP), identified tiny pieces of messenger RNA that are missing in pediatric high-grade glioma tumors but not in normal brain tissues.
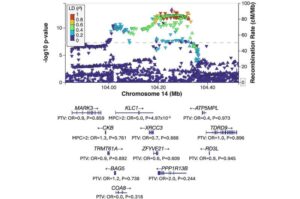
Researchers have discovered eight new genes associated with schizophrenia, in the largest exome-sequencing study of the disorder ever conducted. The breakthrough, made by scientists at the Centre for Neuropsychiatric Genetics and Genomics (CNGG) at Cardiff University, provides new information and improves the understanding and future treatment development for schizophrenia.

The FDA has granted breakthrough device designation to SpinaFX Medical’s Triojection system, a minimally invasive treatment for contained lumbar disc herniations.
MicroPort NeuroScientific has launched its Numen coil embolization system in Egypt through a partnership with PentaMed.
The brain imaging study found that changes in brain dopamine are linked to symptoms of psychosis, no matter whether a person has been diagnosed with schizophrenia, bipolar disorder, or major depression.