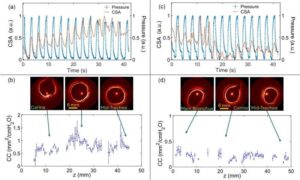
Breathing in 4D: Optical technique maps airway wall elasticity during bronchoscopy
Scientists have developed a faster method for measuring the elasticity of airway walls, a property that can reveal important information about respiratory health.

Scientists have developed a faster method for measuring the elasticity of airway walls, a property that can reveal important information about respiratory health.
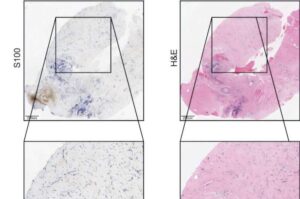
A research team co-led by UCLA investigators has found that pembrolizumab, an immunotherapy drug that helps the immune system attack cancer cells, can effectively shrink or eliminate tumors in patients with unresectable advanced desmoplastic melanoma, a rare and often aggressive form of skin cancer.

Scientists have pinpointed brain activity related to inner speech—the silent monolog in people’s heads—and successfully decoded it on command with up to 74% accuracy.
The immune system is meant to protect the body from infection and disease. But with age, it can become less capable of doing so. However, Mayo Clinic researchers have found that some older people maintain “immune youth”—a new term coined by Mayo researchers to explain a young immune system in someone over age 60.

A smartphone app that delivers real-time, tailored messages may hold the key to helping them quit, according to University of Oklahoma clinical trial results published in JAMA Network Open.
This has been demonstrated by researchers led by Radboud university medical center in a study published in The Lancet Digital Health

Over the past 20 years, a class of cancer drugs called CD40 agonist antibodies have shown great promise—and induced great disappointment.

Scientists at the University of Virginia School of Medicine and the University of Michigan have developed a monoclonal antibody to stop sepsis, a deadly full-body infection.
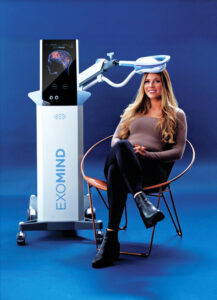
WEST DUNDEE, Ill., Aug. 14, 2025 /PRNewswire/ — Those seeking help with depression or anxiety can now benefit from an FDA-cleared, medication-free procedure at Dundee Dermatology. The center is showcasing EXOMIND, a noninvasive neurostimulation device that enhances mental wellness.

The team used two different AI approaches to design novel antibiotics, including one that showed promise against MRSA.