Fighting aggressive skin cancer becomes possible with AI-designed vaccine approach
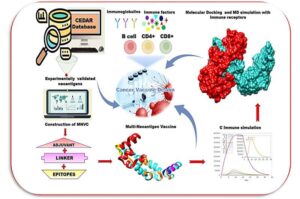
Researchers have harnessed the power of artificial intelligence to design a blueprint for building a vaccine that aims to teach the body’s immune system to fight cancer.