New surgical method effective for groin hernia in women, study shows
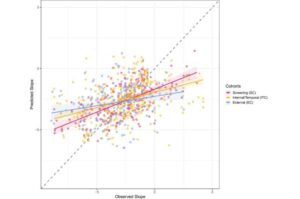
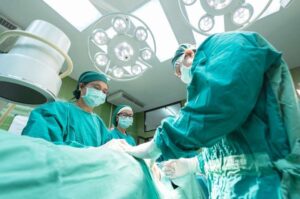
In a study conducted in Uganda and published in JAMA Surgery, researchers from Karolinska Institutet evaluated a new surgical method for treating groin hernias in women. The method could become an alternative in resource-limited settings where laparoscopic techniques are not generally available.