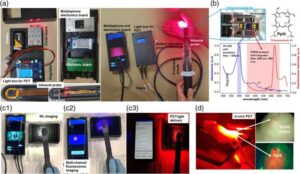
FastWave Medical Garners ‘Fierce 15’ Honor Amid Eighth U.S. Patent Win for IVL Innovation
The recognition underscores FastWave’s rising prominence in intravascular lithotripsy, as the company expands its intellectual property portfolio and momentum in cardiovascular innovation.