
Johnson & Johnson MedTech gets updated FDA nod for Varipulse PFA
Johnson & Johnson MedTech (NYSE: JNJ)+
today announced FDA approval for an update to its Varipulse platform’s irrigation flow rate.

Johnson & Johnson MedTech (NYSE: JNJ)+
today announced FDA approval for an update to its Varipulse platform’s irrigation flow rate.

LivsMed today introduced Stark, its new telesurgery-native robotic system for remote surgery in partnership with Sovato.
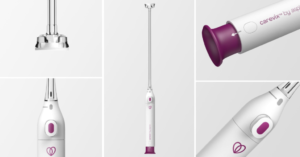
ASPIVIX has announced a new partnership with AGHealth to launch Carevix, its MHRA-approved non-traumatic suction cervical stabiliser, in the United Kingdom. The company says this milestone marks a significant advance in UK women’s healthcare, offering a modern and clinically validated alternative to the traditional cervical tenaculum.

A new device combining ultrasound and advanced imaging to provide crucial information for the safe delivery of drugs into the brain has been developed by University of Queensland researchers. The research is published in the Journal of Controlled Release.
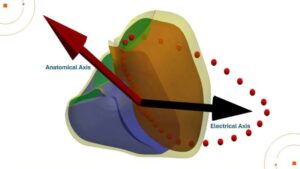
A study led by scientists at King’s has revealed how the physical orientation of the heart inside the chest dramatically influences the electrical signals captured in an electrocardiogram (ECG)—a discovery that could pave the way for more personalized and accurate heart diagnostics.

Approximately 40% of the European population are allergic to pollen, and their symptoms cause an estimated loss of 100 million school and workdays every year.
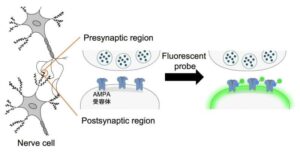
There are few scientific methods more elegantly simple than “just sprinkle it on top.” Researchers at Tohoku University and Nagoya University developed a fluorescent probe that can quickly show synapses, the connection points between brain cells.

A new bionic knee allows amputees to walk faster, climb stairs more easily, and adroitly avoid obstacles, researchers reported in the journal Science.
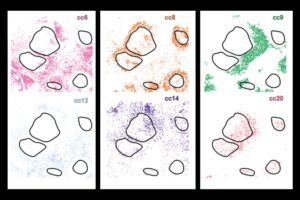
CellLENS reveals hidden patterns in cell behavior within tissues, offering deeper insights into cell heterogeneity — vital for advancing cancer immunotherapy.

TEL AVIV, Israel, July 10, 2025 /PRNewswire/ — Scopio Labs, a trailblazer in Full-Field Digital Cell Morphology, today announced another significant milestone: its fourth U.S. Food and Drug Administration (FDA) clearance (K243144). This latest clearance introduces powerful new Decision Support System (DSS) features to its X100 and X100HT platforms and Peripheral Blood Smear (PBS) Application, further transforming the way laboratories analyze red blood cell (RBC) morphology and identify platelet clumps in peripheral blood smears.