Implantable device could save diabetes patients from dangerously low blood sugar
The new implant carries a reservoir of glucagon that can be stored under the skin and deployed during an emergency — with no injections needed.

The new implant carries a reservoir of glucagon that can be stored under the skin and deployed during an emergency — with no injections needed.
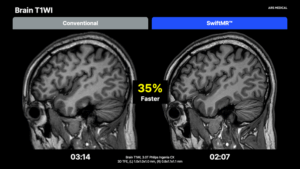
SEOUL, South Korea, July 8, 2025 /PRNewswire/ — AIRS Medical, a leader in AI solutions for diagnostic imaging, has received Medical Device Regulation (MDR) certification from the European Union (EU) for SwiftMR across all body parts. This expanded certification facilitates broader clinical use of SwiftMR in Europe — giving providers new tools to care for a wider range of patients.

Oxiplex is the only FDA authorized intraoperative gel indicated as an adjunct to lumbar spinal surgery to reduce postoperative leg pain and neurological symptoms.
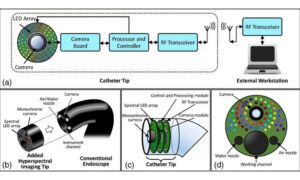
Gastrointestinal cancers remain among the most common forms of cancer. While endoscopy has become a cornerstone of cancer screening and diagnosis over the past two decades, the procedure still misses approximately 8% to 11% of tumors, due to visibility limitations. Now, researchers have developed a prototype imaging system that could significantly improve doctors’ ability to detect cancerous tissue during endoscopic procedures.
Georgia Tech engineers have created a pill that could effectively deliver insulin and other injectable drugs, making medicines for chronic illnesses easier for patients to take, less invasive, and potentially less expensive.
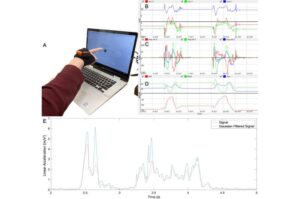
It can take as long as 18 months for children with suspected autism spectrum or attention-deficit-hyperactivity disorders to get a diagnostic appointment with a psychiatrist in Indiana. But an interdisciplinary team led by an Indiana University researcher has developed a new diagnostic approach using artificial intelligence that could speed up and improve the detection of neurodivergent disorders.
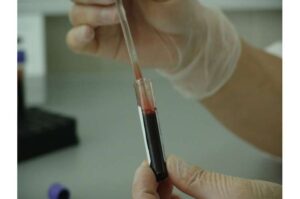
Liquid biopsies are tests that detect signs of cancer through a simple blood draw. Unlike traditional biopsies, which require removing a piece of tissue, a liquid biopsy typically looks for mutations or modification changes in fragments of DNA from cancer cells circulating in the blood.
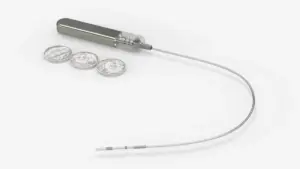
Glucotrack, which is developing a glucose monitor that is implanted through a minimally invasive surgery, said the small study met safety and performance goals.
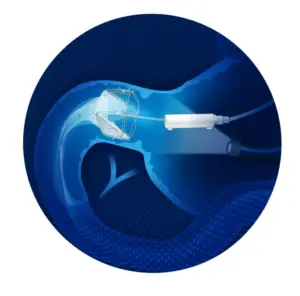
Regulatory milestone paves the way for expanded access to Morphic’s non-implant, incisionless solution for treating acid reflux and related gastrointestinal disorders across Europe.

Mendaera has received FDA 510(k) clearance for its Focalist handheld robotic system, which enhances the precision of ultrasound-guided needle placement across multiple specialties.