
Thermo Fisher’s NGS-Based Oncomine Dx Target Test Gains FDA Approval
FDA clears assay as companion diagnostic for ZEGFROVY™ and broad tumor profiling, advancing precision oncology diagnostics.

FDA clears assay as companion diagnostic for ZEGFROVY™ and broad tumor profiling, advancing precision oncology diagnostics.

Ceramic-based hip resurfacing implant gains European regulatory approval, offering patients a bone-preserving, metal-free alternative for hip joint restoration.

Camgenium, a leading medical device software company, has announced that its software product, ‘Reassure Pregnancy’, can improve outcomes for pregnant women at risk of pre-eclampsia through at-home monitoring.

Dual AI-Powered Imaging Software Set to Enhance Diagnostic Confidence and Workflow Efficiency in MRI
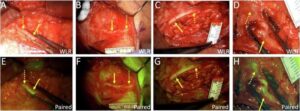
When surgeons dissect tissue to remove a tumor or make a repair, they must work cautiously, relying on electrophysical monitors and their own anatomical knowledge to avoid cutting nerves, which could complicate the patient’s recovery.

A group from Nagoya University in Japan has developed a simple, accurate, and sensitive method for measuring polysialic acid, a unique acidic glycan found in the brain. Polysialic acid fluctuates in the blood of patients with psychiatric disorders.

A new study from Karolinska Institutet shows that artificial intelligence (AI) combined with portable digital microscopy improves the detection of intestinal worm infections, so-called soil-transmitted helminth (STH) in resource-limited settings.
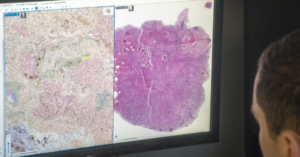
PathAI, a global leader in artificial intelligence (AI) and digital pathology solutions, has announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for AISight Dx—its digital pathology image management system—for use in primary diagnosis in clinical settings.

LEATHERHEAD, England, July 3, 2025 /PRNewswire/ — MatOrtho® is proud to announce that the ReCerf® Hip Resurfacing Arthroplasty (HRA) has received its CE mark, confirming compliance with European safety and performance standards. This important milestone expands access to hip resurfacing, enabling wider availability across the UK, Europe and internationally where CE marking supports market access.

In a recent conversation with MedTech Spectrum, Trent Reutiman, CEO of ANACONDA Biomed, shared insights into the company’s CE Mark approval for the ANA5 Funnel Catheter—a next-generation device designed to improve clot capture and procedural outcomes in acute ischemic stroke treatment.