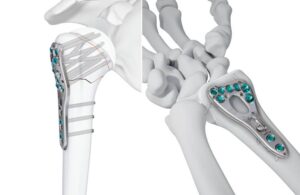
Johnson & Johnson MedTech launches new Volt plating systems for radius, humerus
Johnson & Johnson MedTech (NYSE: JNJ)+
announced today that it launched new variable angle optimized locking technology (VOLT) plating systems.

Johnson & Johnson MedTech (NYSE: JNJ)+
announced today that it launched new variable angle optimized locking technology (VOLT) plating systems.
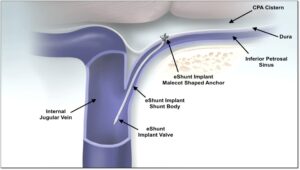
BOSTON, June 18, 2025 /PRNewswire/ — CereVasc, Inc., a clinical-stage medical device company developing novel treatments for neurological diseases, announced today that it has received Investigational Testing Authorization (ITA) from Health Canada to conduct the STRIDE trial, a clinical study evaluating CereVasc’s eShunt System as a treatment for normal pressure hydrocephalus (NPH).
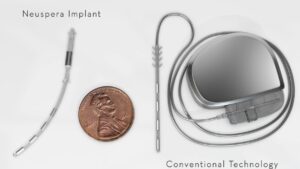
SAN JOSE, Calif., June 18, 2025 /PRNewswire/ — Neuspera® Medical, Inc., the leading developer of integrated technologies powering the future of neuromodulation, today announced that the U.S. Food and Drug Administration (FDA) has approved its integrated sacral neuromodulation (iSNM) system for the treatment of urinary urge incontinence (UUI).
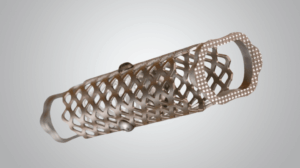
MOUNTAIN VIEW, Calif., June 18, 2025 /PRNewswire/ — Expanding Innovations™ (EI), a rapidly growing spine company specializing in NON-SCREW-based Expandable Technology, today announced U.S. FDA 510(k) clearance for its N-GAGE™ Lumbar Plate System—the company’s first spinal fixation platform as it continues to broaden its procedure-based solutions.
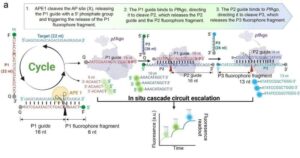
Scientists from the National University of Singapore (NUS) have developed NAPTUNE (Nucleic Acids and Protein biomarkers Testing via Ultra-sensitive Nucleases Escalation), a point-of-care assay that identifies trace amounts of disease-related genetic material, including nucleic acid and protein markers, in less than 45 minutes. Importantly, it accomplished this without the need for laboratory equipment or complex procedures.
University of Alberta researchers have created a new tool to help doctors and patients recognize other illnesses commonly associated with inflammatory bowel disease (IBD), including autoimmune, mental health and heart problems.

New ChoiceSpine™ App Enhances Precision and Efficiency in Spine Surgery with Op.n™ Robotic and Navigation Technology

New technology isolates extracellular vesicles from blood to enable earlier, non-invasive disease detection and personalized treatment strategies.
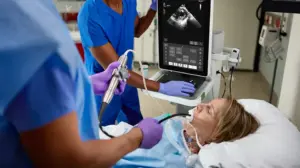
Next-gen portable ultrasound system combines speed, image clarity, and AI-powered tools to elevate diagnostic confidence at the bedside.
By delivering an HIV vaccine candidate along with two adjuvants, researchers showed they could generate many more HIV-targeting B cells in mice.