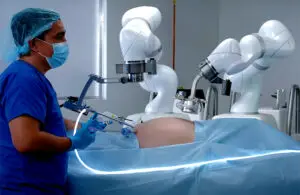
FDA grants expanded clearance for Levita Magnetics surgical robot
Levita Magnetics announced today that it received expanded FDA 510(k) clearance for its MARS (magnetic-assisted robotic surgery) system.

Levita Magnetics announced today that it received expanded FDA 510(k) clearance for its MARS (magnetic-assisted robotic surgery) system.

Anaconda Biomed announced today that it received CE mark approval to commercialize its ANA5 funnel catheter in Europe.

Beau Monde Health and Wellness is the First Medical Spa and Wellness Clinic in Kansas City to Offer the LIPOcel Treatment, a Non-Surgical, Non-Invasive, Quick 30-minute Treatment that Destroys Stubborn Fat. No Downtime, Virtually Pain Free and delivers Permanent Results.
Expanded bio-integrative, metal-free implant line aims to improve outcomes in orthopedic and sports medicine procedures

The launch of Ruby® XL represents a breakthrough in vascular embolization, offering physicians unprecedented flexibility and efficiency for complex case management.

Corvia Medical announced today that it closed a $55 million funding round to support the advancement of its Corvia atrial shunt.
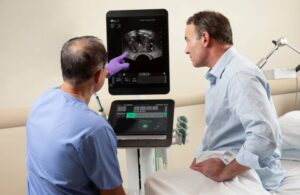
GE HealthCare (Nasdaq: GEHC)+
announced that it launched its bkActiv S series, which it labeled as the next generation of intraoperative ultrasound.

Brain Navi Biotechnology announced today that it received FDA 510(k) clearance for its NaoTrac stereotaxic guiding surgical robotic device.
COPENHAGEN, Denmark, June 16, 2025 /PRNewswire/ — CathVision, a leader in advanced cardiac electrophysiology (EP) technology, has developed a new algorithmic tool (PFAnalyzer) to help physicians assess durable lesion during Pulsed Field Ablation (PFA).
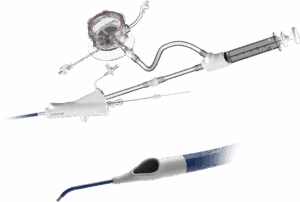
Inquis Medical announced today that the FDA granted expanded 510(k) clearance for its Aventus thrombectomy system.