
Reflow Medical’s Spur® Stent System Receives FDA De Novo Clearance
First-of-its-Kind Retrievable Stent Gains Approval for Treating Challenging Below-the-Knee Peripheral Artery Disease

First-of-its-Kind Retrievable Stent Gains Approval for Treating Challenging Below-the-Knee Peripheral Artery Disease
Mass General Brigham researchers are shining a powerful new light into the viral darkness with the development of Luminescence CAscade-based Sensor (LUCAS), a rapid, portable, highly-sensitive diagnostic tool for processing complex biological samples.
Terumo Interventional Systems has announced the early commercial availability of its FDA-approved Roadsaver Carotid Stent System.
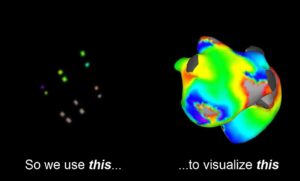
CoreMap announced that it received FDA investigational device exemption (IDE) to extend its electrophysiology (EP) mapping trial to the U.S.
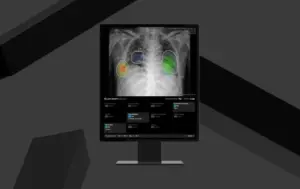
Advanced AI-Powered Chest X-ray Solution Expands Diagnostic Precision and Gains European Regulatory Approval.
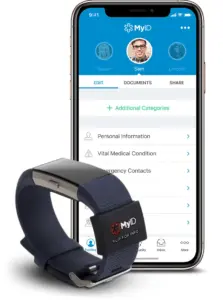
Pioneering collaboration delivers a wrist-worn solution providing instant, secure access to personal medical data—transforming emergency response and everyday care
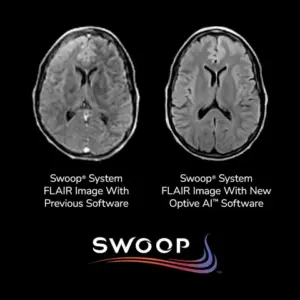
New AI-driven software delivers unprecedented image quality improvements, enhancing diagnostic accuracy and expanding the capabilities of portable MRI technology.
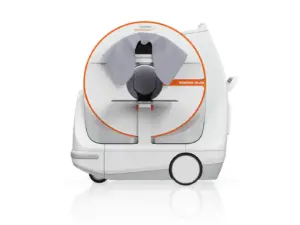
Revolutionary SOMATOM On.site Technology Brings Rapid, Hospital-Grade Stroke Diagnosis Directly to Patients in the Field
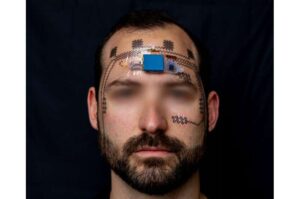
Researchers gave participants face tattoos that can track when their brain is working too hard. Published in the journal Device, the study introduces a non-permanent wireless forehead e-tattoo that decodes brainwaves to measure mental strain without bulky headgear.
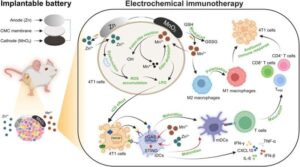
A pioneering biobattery has been shown to reduce tumor growth in the body and could hold the key to a new drug-free immunotherapy treatment in cancer patients.