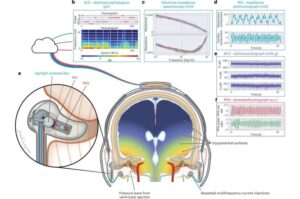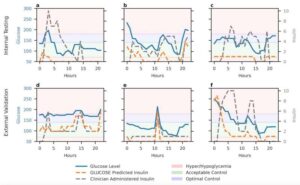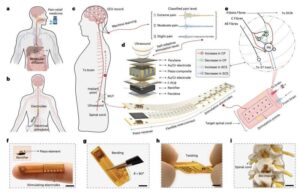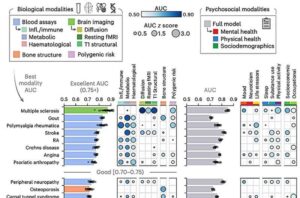
Machine learning finds combined biological and psychosocial data improve chronic pain prediction
Researchers at McGill University and other institutes recently carried out a study aimed at identifying biomarkers and psychosocial factors associated with the development of chronic pain conditions. Their findings, published in Nature Human Behavior, were obtained by analyzing data from a large biomedical database, namely the UK Biobank, using advanced machine learning techniques.