
Aevice wins FDA nod for smart wearable stethoscope for pediatric patients
Aevice Health announced today that it received FDA 510(k) clearance for its AeviceMD smart wearable stethoscope device.

Aevice Health announced today that it received FDA 510(k) clearance for its AeviceMD smart wearable stethoscope device.

ATLANTA, May 13, 2025 /PRNewswire/ — KNoW Biological has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA). This designation is an FDA program designed to expedite the review of devices and medications for serious or life-threatening conditions where there’s preliminary evidence suggesting substantial improvement over existing therapies on the market.

Wearable technologies are revolutionizing health care, but design limitations in adhesive-based personal monitors have kept them from meeting their full potential

A patient-first solution redefines comfort and accuracy in sleep disorder testing across the U.S.
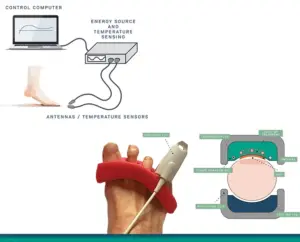
Innovative high-frequency energy technology aims to revolutionize onychomycosis care with a drug-free, patient-friendly solution.

Align Technology’s non-invasive orthodontic innovation set to transform pediatric care in China.

Virtuoso Surgical CEO Duke Herrell took to LinkedIn to announce successful first-in-human clinical cases with the company’s robotic endoscopy system.
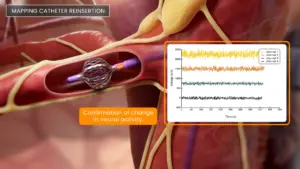
Autonomix Medical (Nasdaq:AMIX) announced that it received a new patent titled controlled sympathectomy and micro-ablation systems and methods.
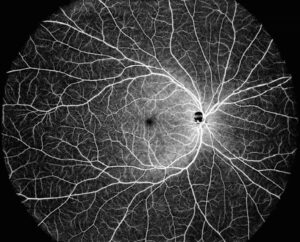
SAN JOSE, Calif. and SHANGHAI, May 15, 2025 /PRNewswire/ — Intalight™, a company that develops advanced ophthalmic technologies and a leader in Optical Coherence Tomography (OCT) today announced it has received CE mark for its DREAM OCT™ platform.
Researchers from the Keck School of Medicine of USC have developed a blood test that can identify early signs of Alzheimer’s disease by measuring proteins linked to the condition. The new test, known as Penta-Plex Alzheimer’s Disease Capture Sandwich Immunoassay (5ADCSI), detects five biomarkers simultaneously, which is more than existing blood tests and runs on equipment commonly used in many laboratories.