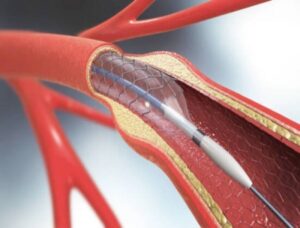
Terumo Neuro Receives FDA Approval for Carotid Stent System
First Dual-Layer Micromesh Carotid Stent Approved for Use in the U.S.

First Dual-Layer Micromesh Carotid Stent Approved for Use in the U.S.
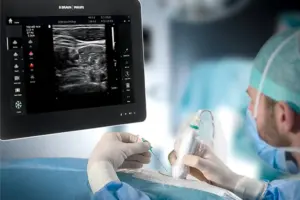
BETHLEHEM, Pa., April 14, 2025 /PRNewswire/ — B. Braun Medical Inc. (B. Braun), a leader in smart infusion therapy and pain management, announced today the launch of the EZCOVER® Probe Cover Set into the U.S. market.
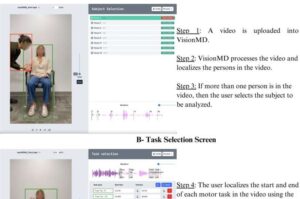
A University of Florida researcher has developed an open-source computer program that uses artificial intelligence to analyze videos of patients with Parkinson’s disease and other movement disorders. The tool, called VisionMD, helps doctors more accurately monitor subtle motor changes, improving patient care and advancing clinical research.
Even though the virus was first discovered in laboratory monkeys in 1958, the original source came from a squirrel.
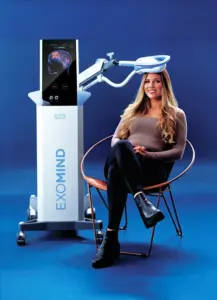
Non-Invasive TMS Device Aims to Enhance Mental Health by Targeting Neural Activity with Magnetic Pulses

Dexcom (Nasdaq: DXCM) today announced a significant regulatory milestone for its continuous glucose monitoring (CGM) technology.

Intuitive (Nasdaq: ISRG) announced today that it received FDA clearance for its fully wristed SP SureForm 45 stapler.
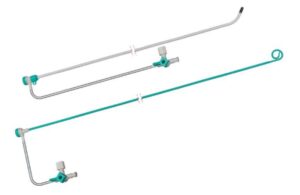
Merit Medical Systems (Nasdaq:MMSI) announced that it launched the Ventrax delivery system for cardiac interventions.
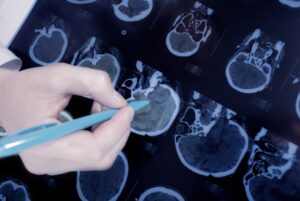
Findings reveal opportunities for new treatments of Parkinson’s and related neurodegenerative disorders

Innovative System Aims to Streamline PCR Testing Workflow and Enhance Laboratory Efficiency