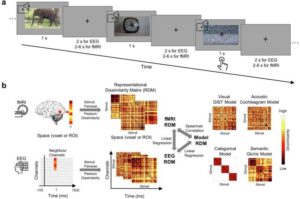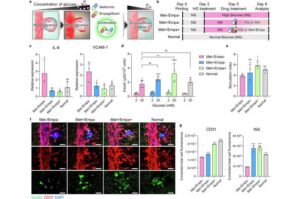
Diabetes treatment approach replicates pancreatic functions with bioink and 3D bioprinting technology
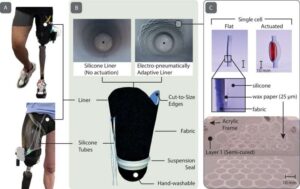
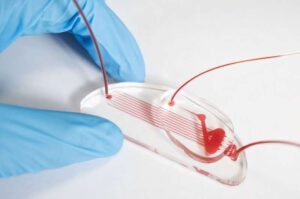
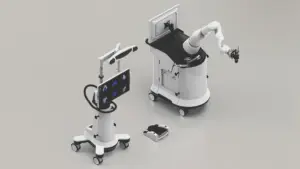
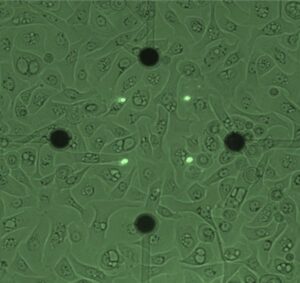
A research team has successfully developed an innovative platform for diabetes treatment using bioink derived from pancreatic tissue and 3D bioprinting technology. This study was recently published online in Nature Communications. The team was led by Professor Jinah Jang at Pohang University of Science and Technology (POSTECH), along with Myungji Kim, a Ph.D. candidate.