
Lifeward wins FDA nod for next-gen exoskeleton
Lifeward (Nasdaq:LFWD) announced today that it received FDA 510(k) clearance for its latest-generation personal exoskeleton device, ReWalk 7.

Lifeward (Nasdaq:LFWD) announced today that it received FDA 510(k) clearance for its latest-generation personal exoskeleton device, ReWalk 7.

Spineart and eCential Robotics today announced the receipt of FDA 510(k) clearance for the use of an application for robotic navigation.
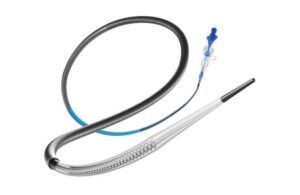
Perfuze announced today that it received FDA 510(k) clearance for its Zipline access catheter and secured significant funding.
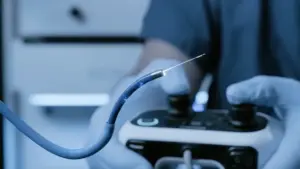
Using Nvidia and GE Healthcare technology, the robotic surgery system is intended to help doctors detect lung cancer earlier.
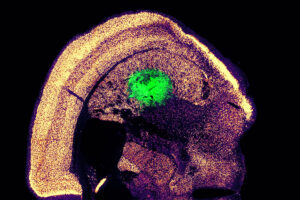
A new, highly efficient process for performing this conversion could make it easier to develop therapies for spinal cord injuries or diseases like ALS.
A researcher at Northeastern University has found a groundbreaking new way to diagnose people with sleep apnea that could open the door for mass screenings of a sleep disorder that affects millions of people.
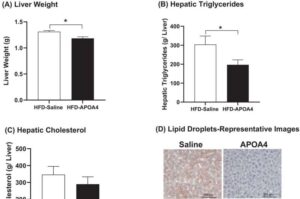
A team of Ohio University researchers have made a groundbreaking development in the fight against obesity. In a study recently published in Obesity, the researchers highlight a protein that is naturally produced in the body but could potentially be applied to combat weight gain and improve metabolic health in patients with obesity.
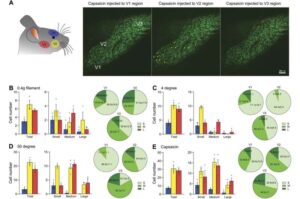
A groundbreaking study has uncovered new insights into the pain pathways associated with temporomandibular joint (TMJ) disorders. Using an innovative in vivo imaging tool to capture functional activity in mouse models of TMJ injury and inflammation, the discovery could pave the way for more effective treatments for the millions affected by TMJ-related facial pain.
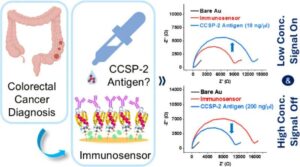
No one looks forward to a colonoscopy. The procedure, which is used to screen for colorectal cancers, is unpleasant and costly and can lead to medical complications. But screening for the cancer is critical; colon cancer is the second leading cause of cancer-related death in the U.S., according to the National Cancer Institute.

A technology developed by the Brazilian company brain4care has been shown to be able to measure absolute values of intracranial pressure (ICP) more accurately than existing non-invasive methods. This is the result of a study published in the journal npj Digital Medicine by researchers from the University of São Paulo in Brazil, the University of Cambridge in the United Kingdom, Emory University in the United States, and the company itself.