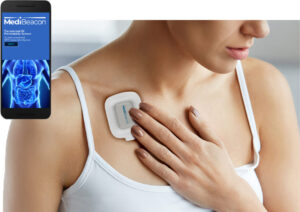
ST. LOUIS, Feb. 25, 2025 /PRNewswire/ — MediBeacon Inc. today announced the National Medical Products Administration (NMPA) in China has approved the MediBeacon® TGFR Monitor and TGFR Sensor for the assessment of kidney function in patients with normal or impaired renal function. Lumitrace® (relmapirazin) injection, categorized as a drug in China, is under review and is targeted for approval in late 2025. The transdermal GFR technology includes Lumitrace (relmapirazin) injection, a non-radioactive, non-iodinated fluorescent GFR tracer agent, which together with the TGFR Monitor and TGFR Sensor allow assessment of kidney function by measuring the clearance rate of the fluorescent agent as it leaves the body.