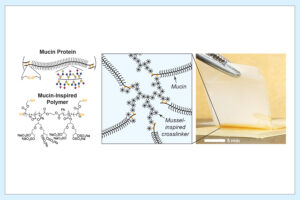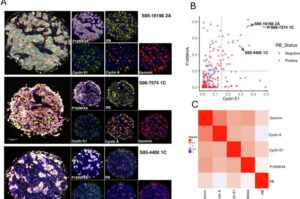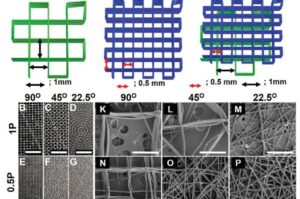
Next-generation degradable 3D meshes offer hope for pelvic organ prolapse repair
A debilitating condition affecting 1 in 4 women is desperately crying out for a solution, and the next generation of treatments to repair the damage of pelvic organ prolapse (POP) has just come a big step closer.