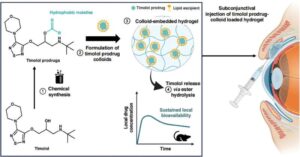
New drug delivery method promises long-lasting glaucoma relief
Researchers at the University of Toronto see a future where a single injection under the eyelid could replace months of daily eye drops to treat glaucoma, a leading cause of blindness.

Researchers at the University of Toronto see a future where a single injection under the eyelid could replace months of daily eye drops to treat glaucoma, a leading cause of blindness.

ETH researchers have developed a new gene switch that can be activated using a commercially available nitroglycerine patch applied to the skin. One day, researchers want to use switches of this kind to trigger cell therapies for various metabolic diseases.
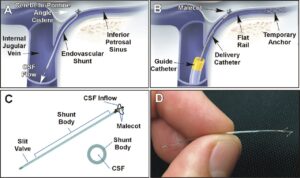
BOSTON, Feb. 13, 2025 /PRNewswire/ — CereVasc, Inc., a clinical-stage medical device company developing novel treatments for neurological diseases, announced today that it has received approval from Argentina’s National Administration of Drugs, Food and Medical Devices (ANMAT) to initiate the STRIDE trial, a clinical study evaluating CereVasc’s eShunt System as a treatment for normal pressure hydrocephalus (NPH).

The U.S. Senate voted 52–48 today to confirm Robert F. Kennedy Jr. as the new secretary of the U.S. Department of Health and Human Services.

Allurion Technologies (NYSE:ALUR) today announced the relaunch of its Allurion Balloon in France following regulatory clearance to resume sales.
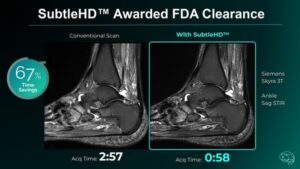
With FDA clearance of SubtleHD™, Subtle Medical launches Subtle-ELITE™, an industry-first AI package designed to achieve superior image quality, unprecedented speed, and workflow automation

Professor Seunghyup Yoo’s research team in the Department of Electrical and Electronic Engineering developed the low-power, high-speed wearable CO2 sensor capable of stable breathing monitoring in real time.
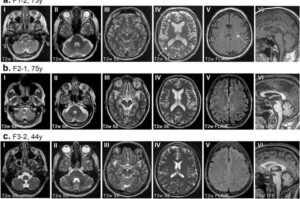
A team led by University of Pittsburgh School of Public Health geneticists has shown, for the first time, that a gene “silencer” that resides in junk DNA is directly sparing people from a devastating and fatal progressive neurological disease.

VADUZ, Liechtenstein, Feb. 12, 2025 /PRNewswire/ — Implantica AG (publ.), a medtech company at the forefront of introducing advanced technology into the body, including a unique device RefluxStop™ for the treatment of acid reflux, a treatment field with 1 billion sufferers, is pleased to announce that the FDA has accepted Module 1 of the company’s premarket approval (PMA) application, and that this module is now considered closed.
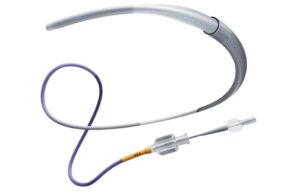
Johnson & Johnson MedTech (NYSE: JNJ)+
announced today that it launched the CereGlide 92 catheter system for treating acute ischemic stroke.