
Sinocare prevails in CGM trademark spat with Abbott
Sinocare announced recently that it successfully defended itself in court against trademark infringement claims brought by Abbott (NYSE:ABT).

Sinocare announced recently that it successfully defended itself in court against trademark infringement claims brought by Abbott (NYSE:ABT).

The company announces CE MDR approval for its novel Delphi-MD neurodiagnosis medical device in preparation for EU commercialization, improving patients’ clinical outcomes and well being while reducing healthcare systems and payors’ financial burden.

A team of UC Berkeley engineers from the Embodied Dexterity Group has developed a wearable device to enhance grasping functionality in this population. Dubbed the Dorsal Grasper, this assistive device leverages voluntary wrist extension and uses supernumerary robotic fingers on the back of the hand to facilitate human-robot collaborative grasping.

Researchers at the LKS Faculty of Medicine of the University of Hong Kong (HKUMed) have invented an oral formulation of arsenic trioxide (Oral-ATO; ARSENOL) for the treatment of acute promyelocytic leukemia (APL), a blood cancer that once had a high fatality rate.
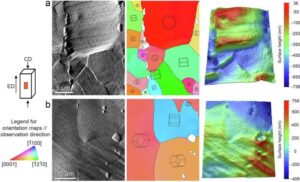
Monash research could transform how broken bones are treated, with the development of a special zinc-based dissolvable material that could replace the metal plates and screws typically used to hold fractured bones together.

Researchers have achieved a breakthrough in wearable health technology by developing a novel self-healing electronic skin (E-Skin) that repairs itself in seconds after damage. This could potentially transform the landscape of personal health monitoring.

Georgia Tech researchers have created a 3D-printed heart valve made of bioresorbable materials and designed to fit an individual patient’s unique anatomy. Once implanted, the valves will be absorbed by the body and replaced by new tissue that will perform the function that the device once served.

Relocating manufacturing would require substantial capital investment, but the timespan for the president’s new tariffs is unclear, supply chain experts said.
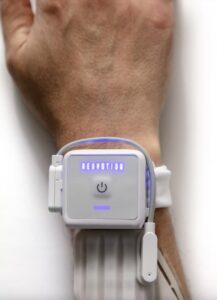
STAMFORD, Conn., Feb. 11, 2025 /PRNewswire/ — Neuvotion, Inc. is an early-stage medical device company developing AI-driven neuromodulation technologies and products for use in the neurorehabilitation, brain-computer interface (BCI), and physical therapy markets. Neuvotion has received FDA 510(k) clearance for their first product, NeuStim™, a non-invasive, surgery-free wearable that electrically stimulates muscles dynamically and with high-precision.

BOSTON, Feb. 11, 2025 /PRNewswire/ — InnoVoyce, a leading medical technology company, proudly announces its ISO 13485:2016 certification, underscoring its dedication to excellence in the design, manufacturing, installation, and servicing of lasers and fiber devices. Alongside this milestone, the company is thrilled to share that its FDA-cleared VYLO™ 455nm Blue Light Laser System is now available nationwide, offering healthcare professionals an advanced tool for treating laryngeal disorders and a wide range of surgical applications.