Inspire Medical reveals DOJ civil investigation
CEO Tim Herbert told investors the company received a civil investigative demand from the Department of Justice in January for information on marketing and reimbursement practices.

CEO Tim Herbert told investors the company received a civil investigative demand from the Department of Justice in January for information on marketing and reimbursement practices.

Doctors at Queen Mary University of London, Barts Health NHS Trust, and University College London have led the development of a simple, minimally invasive Targeted Thermal Therapy (Triple T) that has the potential to transform medical management of a common, but commonly overlooked, cause of high blood pressure.

A deep neural network called CHAIS may soon replace invasive procedures like catheterization as the new gold standard for monitoring heart health.

Newronika announced today that it received FDA investigational device exemption (IDE) for its deep brain stimulation (DBS) system.
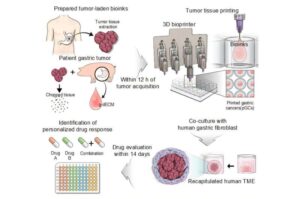
A collaborative research team from POSTECH has successfully developed a gastric cancer model using 3D bioprinting technology and patient-derived cancer tissue fragments. This innovative model preserves the characteristics of actual patient tissues and is expected to rapidly evaluate and predict individual patient drug responses. The research has been published in the journal Advanced Science.

Senseonics (NYSE:SENS) announced today that it filed for CE mark for its Eversense 365 continuous glucose monitor (CGM) system.

CHICAGO, Feb. 6, 2025 /PRNewswire/ — Sibel Health, an award-winning medical technology company that develops advanced wearable sensors, software, and AI/ML algorithms for clinical trials and clinical care, is pleased to announce a major peer-reviewed publication, “Artificial Intelligence-Enabled Wearable Devices and Nocturnal Scratching in Mild Atopic Dermatitis,” in JAMA Dermatology. This research describes a groundbreaking digital health technology designed to measure and reduce nighttime scratching in people with mild atopic dermatitis.
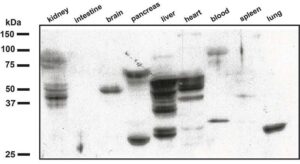
Now researchers at Case Western Reserve University have developed a method to detect inflammation using antibodies, potentially leading to blood tests for disease-specific biomarkers such as for heart disease, Alzheimer’s disease and various cancers. Their breakthrough also holds promise for drug discovery.
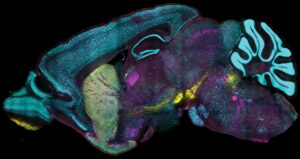
Tissue processing advance can label proteins at the level of individual cells across large samples just as fast and uniformly as in dissociated single cells.

ATLANTA, Feb. 5, 2025 /PRNewswire/ — Huxley Medical, a commercial-stage medical technology firm focused on streamlining detection of sleep and heart disorders, announced that the US Food and Drug Administration (FDA) has cleared the SANSA home sleep apnea test to begin using cellular data upload capabilities. This breakthrough eliminates the need for Bluetooth pairing or smartphone apps commonly required to transmit test data to physicians, addressing a common source of failed home testing, while simplifying the diagnostic process for patients and providers alike.