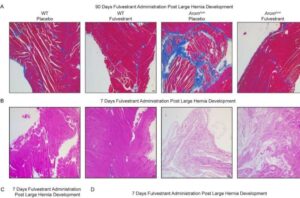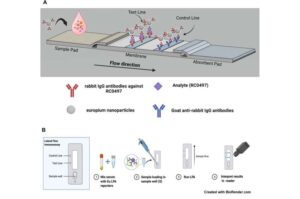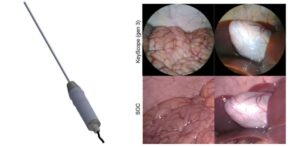
Low-cost laparoscope designed for low- and middle-income countries
Despite its advantages, laparoscopic surgery remains largely inaccessible in low- and middle-income countries (LMICs) due to the high cost of equipment and other logistical challenges. To bridge this gap, researchers recently developed the KeyScope, an affordable laparoscope designed specifically for LMICs. As reported in Biophotonics Discovery, the KeyScope system was developed through an iterative human-centered design approach.