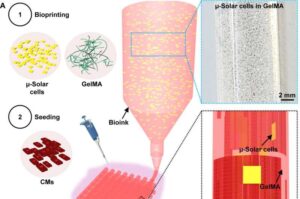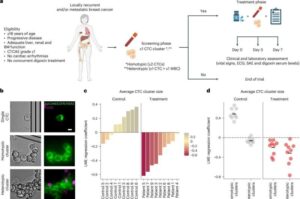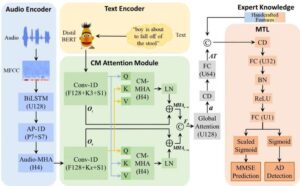
Innovative voice-based approach enables early detection of Alzheimer’s disease
A multi-task learning framework, DEMENTIA, has been developed by Prof. Li Hai and his team at the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, to improve the early detection and assessment of Alzheimer’s disease (AD). The research was recently published in the IEEE Journal of Biomedical and Health Informatics.