
Fire1 raises $120M for breakthrough heart failure management tech
Fire1 announced today that it completed a $120 million financing round to accelerate the advancement of its heart failure management system.

Fire1 announced today that it completed a $120 million financing round to accelerate the advancement of its heart failure management system.

OXNARD, Calif., Jan. 6, 2025 /PRNewswire/ — PreEvnt, a subsidiary of SCOSCHE Industries, a leading innovator of award-winning consumer technology will proudly unveil isaac* by PreEvnt at the Digital Health Exhibit at CES.

Medtronic (NYSE: MDT)+
today announced CE mark approval for its Harmony transcatheter pulmonary valve (TPV) system
PieX AI announced today that it launched a pendant featuring on-device AI for managing mental health through sensing technology.
IceCure Medical (Nasdaq:ICCM) announced today that it received an intention to grant notice from the European Patent Office.

Qalo today unveiled its latest silicone smart ring technology for health tracking, called QRNT (pronounced “current”).
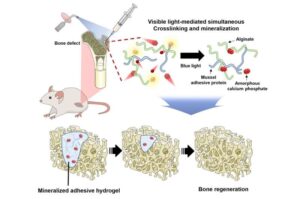
A research team has developed an innovative injectable adhesive hydrogel for bone regeneration. This hydrogel utilizes harmless visible light to simultaneously achieve cross-linking and mineralization without the need for bone grafts. The research was recently published in the journal Biomaterials.
When children receive their second measles-mumps-rubella vaccine, around the time they start kindergarten, they gain protection against all three viruses for all or most of their lives. Yet the effectiveness of an influenza vaccine given in October starts to wane by the following spring.

Researchers at the University of Pittsburgh have developed a nasal swab test for kids that diagnoses specific asthma subtype, or endotype. This non-invasive approach could help clinicians prescribe medications more precisely and pave the way for research toward better treatments for lesser-studied asthma types, which have been difficult to diagnose accurately until now.
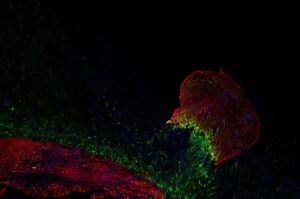
Researchers from the Bakkers group at the Hubrecht Institute have successfully repaired damaged mouse hearts using a protein from zebrafish. They discovered that the protein Hmga1 plays a key role in heart regeneration in zebrafish. In mice, this protein was able to restore the heart by activating dormant repair genes without causing side effects, such as heart enlargement.