Cartessa Aesthetics Introduces Three New Technologies to Give Providers a Head Start for 2025
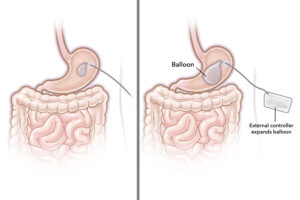
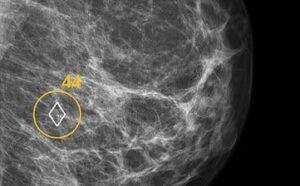
MELVILLE, N.Y., Dec. 4, 2024 /PRNewswire/ — At October’s Inner Circle Invitational in Boca Raton, Cartessa Aesthetics introduced three new technologies to the nearly 400 aesthetic providers in attendance.