LifeSignals Receives FDA 510(k) Approval for UbiqVue™ 2A Multiparameter System
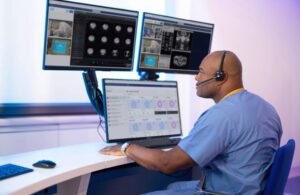
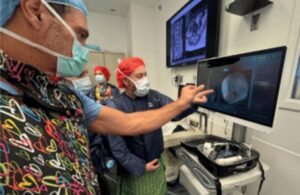
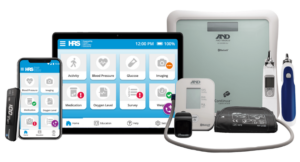
MILPITAS, Calif., Nov. 25, 2024 /PRNewswire/ — LifeSignals, Inc. today announced that the UbiqVue 2A Multiparameter System has received FDA Class II 510(k) clearance, marking a significant milestone in continuous wireless patient monitoring for population health management.