Nanosonics Launches Innovative Wireless Ultrasound Probe Holder for Enhanced Disinfection and Traceability
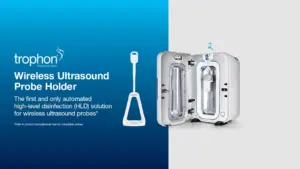
SYDNEY, Nov. 19, 2024 /PRNewswire/ — Nanosonics, Inc., a global leader in infection prevention solutions, is proud to announce the release of its newest accessory for the US and Canadian markets—the trophon® Wireless Ultrasound Probe Holder.