
Distalmotion wins FDA de novo nod for Dexter surgical robot
Distalmotion announced today that it received FDA de novo approval for its Dexter surgical robot for adult inguinal hernia repair.

Distalmotion announced today that it received FDA de novo approval for its Dexter surgical robot for adult inguinal hernia repair.
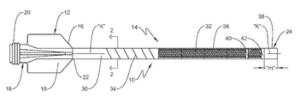
Vantis Vascular announced today that it received FDA 510(k) clearance for its CrossFast integrated microcatheter guide extension system.

Dexcom’s third-quarter earnings report included news of a significant submission to the FDA for the company’s latest-generation technology.
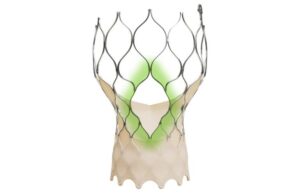
Medtronic (NYSE:MDT) announced today that it received CE mark approval for its Evolut FX+ transcatheter aortic valve implantation (TAVI) system.
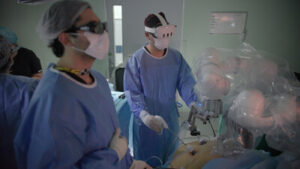
MOUNTAIN VIEW, Calif., Oct. 24, 2024 /PRNewswire/ — In a groundbreaking advancement for medical science, surgeons in Santiago, Chile, performed the world’s first robotic surgeries combining 3D visualization and augmented reality (AR), marking a significant step forward in improving surgical speed, visualization, precision, and patient outcomes.

SEOUL, South Korea, Oct. 24, 2024 /PRNewswire/ — Sky Labs’ CART BP, an innovative blood pressure monitoring device, has been recognized by the International Society of Hypertension (ISH) for its exceptional accuracy in both day and night measurements.

Medtronic (NYSE:MDT) today said it received FDA approval for its Affera mapping and ablation system with the Sphere-9 catheter.

Luna Diabetes says it has launched a pivotal trial for what it describes as “the world’s smallest insulin patch pump.”

The agency cleared one of two 510(k) submissions iRhythm filed for the heart monitor after receiving a warning letter from the agency last year.
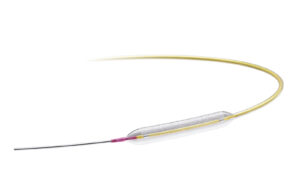
Medtronic (NYSE: MDT)+
announced today that it received FDA investigational device exemption (IDE) for its Prevail drug-coated balloon (DCB).