
Stevanato Group expands large-volume drug delivery offerings
Stevanato Group announced today that it expanded the capacity of its Vertiva on-body device for subcutaneous large-volume drug delivery.

Stevanato Group announced today that it expanded the capacity of its Vertiva on-body device for subcutaneous large-volume drug delivery.

Johnson & Johnson (NYSE: JNJ)+
announced today that it expanded the rollout of its latest Tecnis Odyssey presbyopia-correcting intraocular lenses (PC-IOLs).

Pi-Cardia announced today that the FDA granted clearance for its ShortCut leaflet modification device for TAVR procedures.
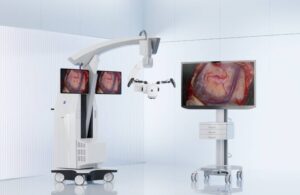
Zeiss Medical Technology announced today that it launched the Kinevo 900 S robotic visualization system for neurosurgery procedures.

ResMed (NYSE: RMD)+
announced today that it launched its new AirTouch N30i continuous positive airway pressure (CPAP) mask.

Olympus Corporation has announced the launch of VISERA S, an all-in-one imaging platform with stroboscopy. The new video platform integrates advanced diagnostic capabilities, including Narrow Band Imaging (NBI) technology, and forms part of the expanding Ear, Nose and Throat (ENT) solution from Olympus.

The system will bolster Medtronic Cardiac Surgery’s portfolio of cardiac surgical and respiratory innovation.
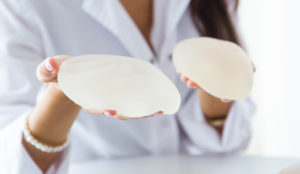
Motiva is the first new breast implant to receive premarket approval since 2013, according to Establishment Labs, and enters a market reshaped by safety issues linked to other products.
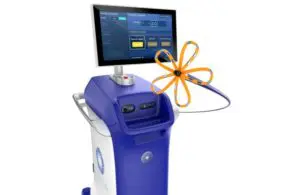
Boston Scientific (NYSE: BSX)+
continues to expand the reach of its Farapulse pulsed field ablation system for treating atrial fibrillation
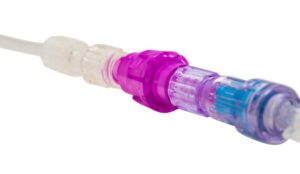
The IV dislodgement-prevention device is now approved for use with blood or blood product transfusions.