Implantica submits FDA PMA application Clinical Module 2 for RefluxStop™ for U.S. market approval
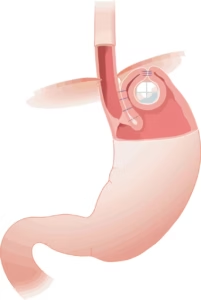
VADUZ, Liechtenstein, Nov. 14, 2024 /PRNewswire/ — Implantica AG (publ.), a medtech company at the forefront of introducing advanced technology into the body, including the unique device RefluxStop™ for the treatment of acid reflux, a treatment field with 1 billion sufferers, announces the submission of the second clinical module of the Premarket Approval (PMA) application to the US FDA for RefluxStop™ along with responses to the FDA’s findings from the first module.