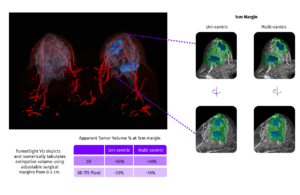
FDA clears SimBioSys clinical decision support tech for breast cancer surgery
SimBioSys announced that it received its second FDA 510(k) clearance for TumorSight Viz to expand its use by breast surgeons in the U.S.

SimBioSys announced that it received its second FDA 510(k) clearance for TumorSight Viz to expand its use by breast surgeons in the U.S.
Medtronic and Recor Medical each announced separate Medicare reimbursement approvals for their respective renal denervation (RDN) technologies.

Akura Medical, a Shifamed portfolio company, announced today that the FDA granted its Katana system investigational device exemption (IDE).

NeurAxis (NYSE:NRXS) announced today that the FDA granted an expanded 510(k) clearance for its IB-Stim non-implanted nerve stimulator.

Front Line Medical Technologies Inc, a leader in medical devices for emergency and trauma care, has announced that its COBRA-OS (Control of Bleeding, Resuscitation, Arterial Occlusion System) has been specially recognised in TIME’s Best Inventions of 2024.

CINCINNATI, Nov. 4, 2024 /PRNewswire/ — Sense Neuro Diagnostics, a leader in developing technology for the diagnosis and monitoring of brain injuries, today announced it has been awarded a $2 million contract by the Department of Defense (DoD).

LAS VEGAS, Nov. 4, 2024 /PRNewswire/ — SeeMedX Inc. (“SeeMedX”) today announced the submission of its 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for its innovative, non-invasive cardiac monitoring device, designed to allow medical professionals to have real-time insights into cardiac performance and fluid status

Quanta Dialysis Technologies announced today that it received FDA 510(k) clearance for the use of its dialysis system in the home.

R3 Vascular announced today that the FDA granted investigational device exemption (IDE) to evaluate its Magnitude drug-eluting bioresorbable scaffold.

iRhythm Technologies (Nasdaq:IRTC) announced that the FDA granted 510(k) clearance for Zio AT design modifications and labeling updates.