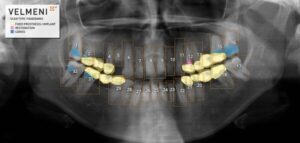
VELMENI® RECEIVES FDA CLEARANCE
SUNNYVALE, Calif., Sept. 3, 2024 /PRNewswire/ — VELMENI announced today it received FDA 510(k) clearance for VELMENI for DENTISTS, an innovative AI that analyzes and annotates dental X-Rays

SUNNYVALE, Calif., Sept. 3, 2024 /PRNewswire/ — VELMENI announced today it received FDA 510(k) clearance for VELMENI for DENTISTS, an innovative AI that analyzes and annotates dental X-Rays

Embecta (Nasdaq:EMBC) today entered the insulin patch pump market with a major regulatory milestone.

Vitestro says it is the first company ever to achieve CE marking for an autonomous blood drawing device.
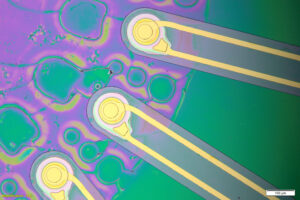
MIT engineers’ implantable ImPULS device could become an alternative to the electrodes now used to treat Parkinson’s and other diseases.

Women are offered the opportunity to benefit from Carevix at the Peterborough City Hospital – North West Anglia NHS Foundation Trust.

Coronary artery disease (CAD) is the most common cause of illness-based death throughout the world. According to the World Health Organization, CAD causes 17.9 million deaths per year worldwide, nearly one-third of all illness-based deaths annually.

Think Surgical announced that the FDA granted 510(k) clearance for its TMINI miniature robotic system for use with Medacta’s knee systems.
The comprehensive cloud-based platform combines AI diagnostics and detailed measurements for rapid and accurate diagnosis of structural heart disease and heart failure.
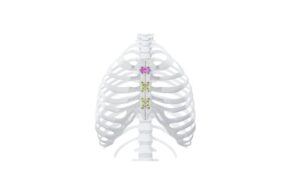
Johnson & Johnson MedTech’s DePuy Synthes announced today that it launched its MatrixSternum plate and screw fixation system.
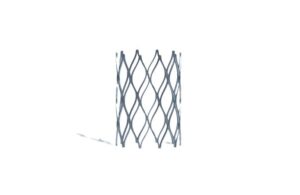
Renata Medical today said the FDA approved its first-of-its-kind Minima growth stent tailored for neonates, infants and young children.