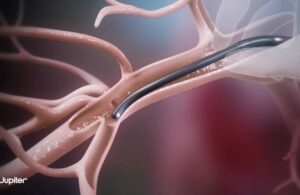
FDA grants IDE for Jupiter Endovascular’s pulmonary embolectomy system
Jupiter Endovascular announced today that the FDA approved an investigational device exemption (IDE) study for its Vertex system.

Jupiter Endovascular announced today that the FDA approved an investigational device exemption (IDE) study for its Vertex system.

CereVasc announced today that it received FDA breakthrough device designation for its eShunt system for treating normal pressure hydrocephalus (NPH).

Neuros Medical announced today that it received FDA approval for its Altius direct electrical nerve stimulation system.
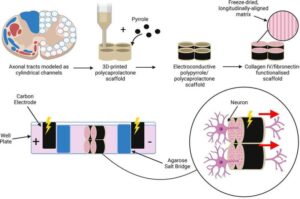
A research team at RCSI University of Medicine and Health Sciences has developed a new implant that conveys electrical signals and may have the potential to encourage nerve cell (neuron) repair after spinal cord injury.

INDIANAPOLIS, Aug. 28, 2024 /PRNewswire/ — Prevounce Health, a leading provider of remote care management software, devices, and services, announces the launch of its first blood oxygen device for remote patient monitoring (RPM): Pylo OX1-LTE.

The new ACURATE Prime valve system is indicated to restore function and normal blood flow through a narrowed aortic valve in low, intermediate and high-risk patients with severe aortic stenosis.
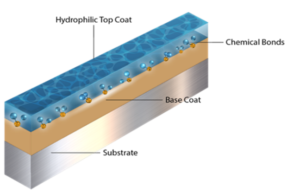
HORSHAM, Pa., Aug. 27, 2024 /PRNewswire/ — Biocoat Incorporated, a leader in the development, manufacturing, and application of hydrophilic coatings for medical devices, is proud to announce the issuance of a patent for “Lumen Coating Method and Apparatus.” This new patent covers a groundbreaking thermal cure hydrophilic coating technology specifically designed for application to the lumen, or inner diameter. This technology is commonly applied in catheters and other medical devices used in multiple medical specialties, including cardiology and neurology.
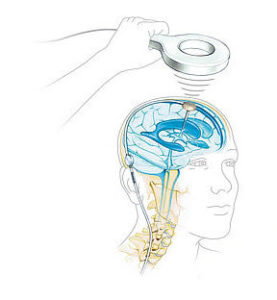
CENTER VALLEY, Pa., Aug. 27, 2024 /PRNewswire/ — Aesculap, Inc. (Aesculap), in partnership with Christoph Miethke GmbH & Co. KG (MIETHKE), announced today that the U.S. Food and Drug Administration (FDA) granted Breakthrough Device Designation for the M.scio® System. This unique, non-invasive, telemetric pressure measurement system is designed to provide continuous access to long-term, intracranial pressure (ICP) monitoring of cerebrospinal fluid (CSF) for the management of hydrocephalus via a permanent, fully implantable sensor.
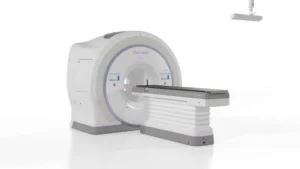
Accuray (Nasdaq: ARAY)+
announced today that it received CE mark approval for its Helix CT-guided helical radiotherapy system.

The Czech company says this is the first non-invasive closed-loop neuromodulation system to treat overactive bladder (OAB) without surgery or drugs.