
Enovis unveils new ankle replacement system
Enovis (NYSE: ENOV)+ announced today that it unveiled its STAR Ankle system with new e+ Polyethylene.

Enovis (NYSE: ENOV)+ announced today that it unveiled its STAR Ankle system with new e+ Polyethylene.
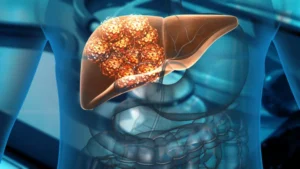
Following a nearly decade-long effort, Delcath Systems finally won the FDA’s greenlight for its Hepzato Kit for the liver-directed treatment of adult patients with metastatic uveal melanoma.
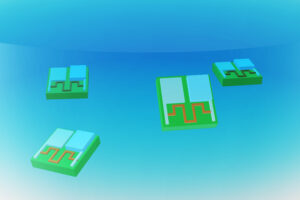
These zinc-air batteries, smaller than a grain of sand, could help miniscule robots sense and respond to their environment.
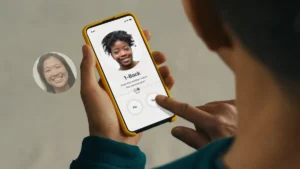
Intended to supplement medications, the six-week, app-based treatment will cost $50 on a cash-pay basis.
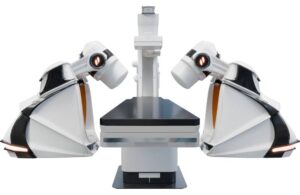
Stereotaxis (NYSE:STXS) announced that it obtained CE mark in Europe and submitted an FDA 510(k) application for its next-generation surgical robot.
Surgical Planning Associates announced today that it received FDA 510(k) clearance for its HipInsight 2.0 mixed-reality guidance system.
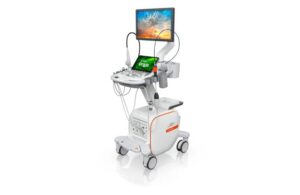
Siemens Healthineers announced today that it received FDA clearance for its Acuson Origin ultrasound system and AcuNav Lumos 4D ICE catheter.
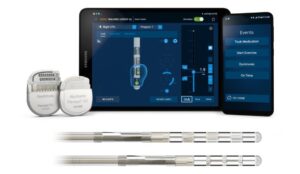
Medtronic says the approval provides more options to meet patients’ personalised needs, including those with Parkinson’s disease.
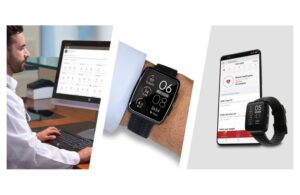
Masimo (Nasdaq: MASI)+
announced today that the FDA granted 510(k) clearance for its W1 medical watch for connectivity purposes

Researchers tested their prototype in a real world setting to assess its accuracy and determine needs for improvement.